Dancing Milk Colors Experiment
Supplies:
Plate
Milk
Food Coloring
Dish Soap
Q-tip
Now Follow These Steps:
Step 1: Fill the plate with enough milk to completely cover the bottom.
Step 2: Add 4 drops of food coloring in a diamond pattern, about an inch away from the center of the plate.
Step 3: Use a q-tip to add dish soap to the very center of the plate.
Step 4: Slowly rotate the q-tip to make the colors dance!
Hold the q-tip in the empty center space.
Remember to help clean up! No crying over spilled milk.
Here’s the Science:
Milk is mostly water, but it also contains vitamins, minerals, proteins, and tiny droplets of fat suspended in solution. Fats and proteins are sensitive to changes in the surface tension of the surrounding solution- the milk!
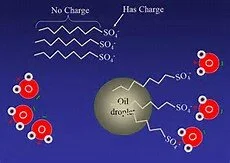
The secret of the bursting colors is in the chemistry of that tiny drop of soap. Like other oils, milk fat is a non-polar molecule. That means it doesn’t like to dissolve in water (a polar molecule). When soap is mixed in, however, the non-polar tail of soap micelles (molecular soap structures in solution) break up and collect the non-polar fat molecules. Then the polar surface of the micelle connects to a polar water molecule with the fat held inside the soap micelle.
Thanks to the soap’s amphipathic connection (having both polar and non-polar sides), the non-polar fat can then be carried away by the polar water.
The uncharged tail of the soap bonds to oils, while the charged head bonds to water molecules.
This is when the fun begins.
The molecules of fat bend, roll, twist, and contort in all directions as the soap molecules race around to join up with the fat molecules. During all of this fat molecule gymnastics, the food coloring molecules are bumped and shoved everywhere, providing an easy way to observe all the invisible activity. As the soap becomes evenly mixed with the milk, the action slows down and eventually stops.
“Try it out with water or other liquids. Do the colors still move around?”
“Try using thin glue instead of milk to make a beautiful piece of art to keep!”
Want to do more experiments? Sign up for Science Camp or Science Club.